The Periodic Table and Chemical Bonding
The Periodic Table
10 Topics | 9 Quizzes
Chemical Bonding and Structures
8 Topics | 7 Quizzes
Ionic and Covalent Compounds
8 Topics | 7 Quizzes
Types of Chemical Reactions
Combination, Decomposition and Displacement Reactions
6 Topics | 5 Quizzes
Oxidation Reactions: Combustion and Corrosion
9 Topics | 8 Quizzes
Acid-Base Reactions
10 Topics | 9 Quizzes
Acid-Metal Reactions
5 Topics | 4 Quizzes
Rates of Chemical Reactions
Rate of a Chemical Reaction
4 Topics | 3 Quizzes
Factors that Affect Rate of Reaction
7 Topics | 6 Quizzes
6 | The Transition Metals

6 | The Transition Metals
The Transition Metals
- The metals in groups 3-12 are known as the transition metals.
- Transition metals generally have the following properties:
- They are hard metals (group 11 is an exception).
- They have high boiling points (except mercury, which is a liquid at room temperature).
- They have high densities.
- They have either one or two valence electrons and form positive ions.
- Many can form ions with different charges (eg, copper can form Cu+ and Cu2+ ions).
- They usually form coloured compounds.
- Metals to the right of the transition metals are known as post-transition metals.
- They have similar properties to transition metals.
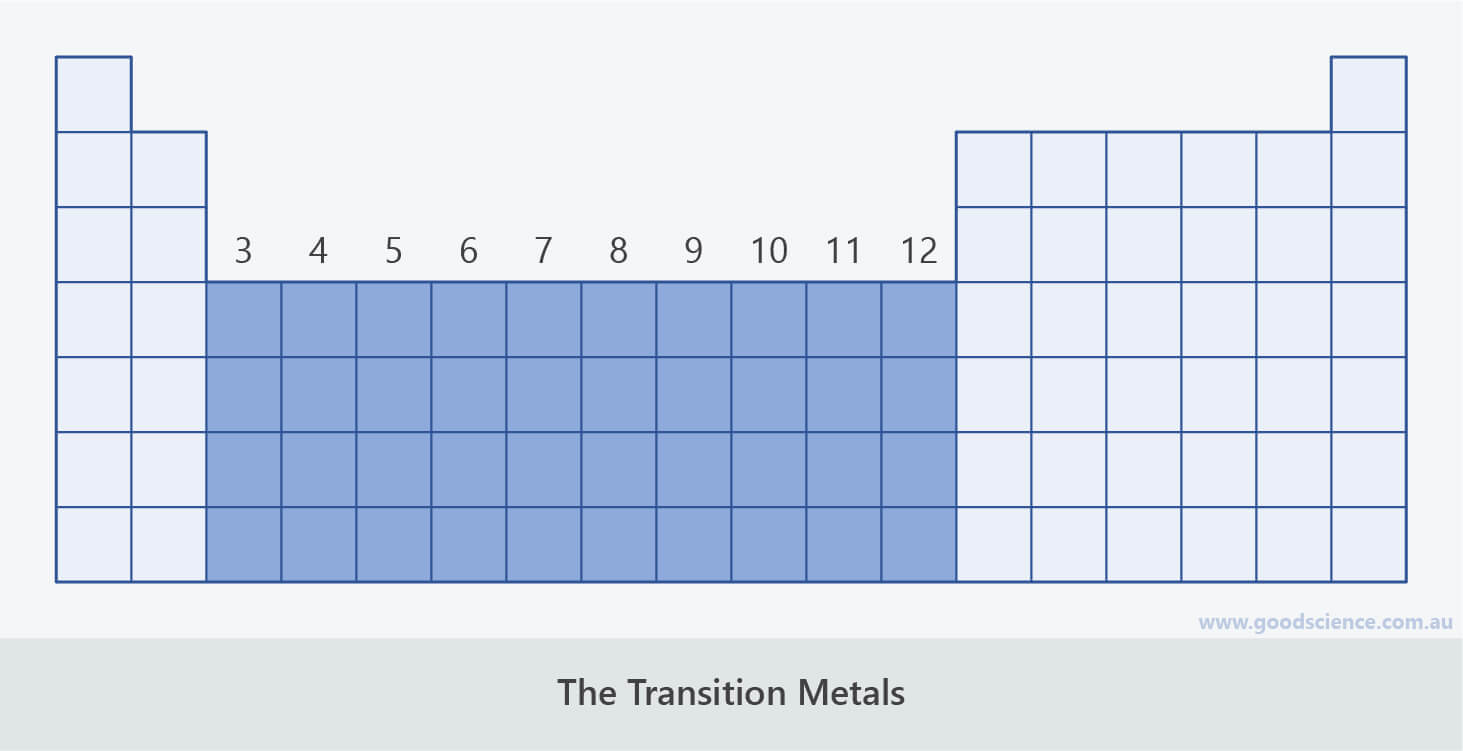
The metals in groups 3-12 are known as the transition metals.
