3 | Electron Configuration and the Periodic Table
Electron Configuration and the Periodic Table
- Since chemical reactivity of elements is related to their electron configuration, there is a correlation between the electron configuration of an element and its location in the periodic table.
- The number of the period that an element is in is the same as the number of electron shells in its atoms.
- All elements in a particular period will therefore have the same number of electron shells.
- For example, potassium (K), iron (Fe) and bromine (Br) are all in period 4. Therefore their atoms all contain four electron shells.
- For main group elements, the number of the group that an element is in reflects the number of valence electrons (electrons in the outer shell) in its atoms.
- The number of valence electrons is equal to the last digit of the group number. (In the older group numbering system mentioned above, the number of valence electrons is equal to the group number.)
- All elements in a particular group will therefore have the same number of electron shells.
- For example, fluorine (F), chlorine (Cl) and iodine (I) are all in group 17 (group VII). Therefore their atoms all contain seven valence electrons.
- Since the location of main group elements can be used to determine the number of electron shells and the number of valence electrons, it can also be used to determine the electron configuration, and vice versa.
- Example 1
- Magnesium is in period 3 and group 2 of the periodic table.
- Therefore magnesium atoms have three electron shells and two electrons in the valence shell.
- Since the maximum number of electrons in the first shell is two and the maximum number of electrons in the second shell is eight, magnesium has the electron configuration 2,8,2.
- Example 2
- Barium has the electron configuration 2, 8, 18, 18, 8, 2.
- This shows that barium atoms have six electron shells (because there are six numbers) and two electrons in the valence shell (because the last number is 2).
- Therefore barium is in period 6 and group 2 of the periodic table.
- For transition metals, lanthanides and actinides, electron configuration is complex and the number of valence electrons cannot be predicted from the location of the element in the periodic table, but is usually 1 or 2.
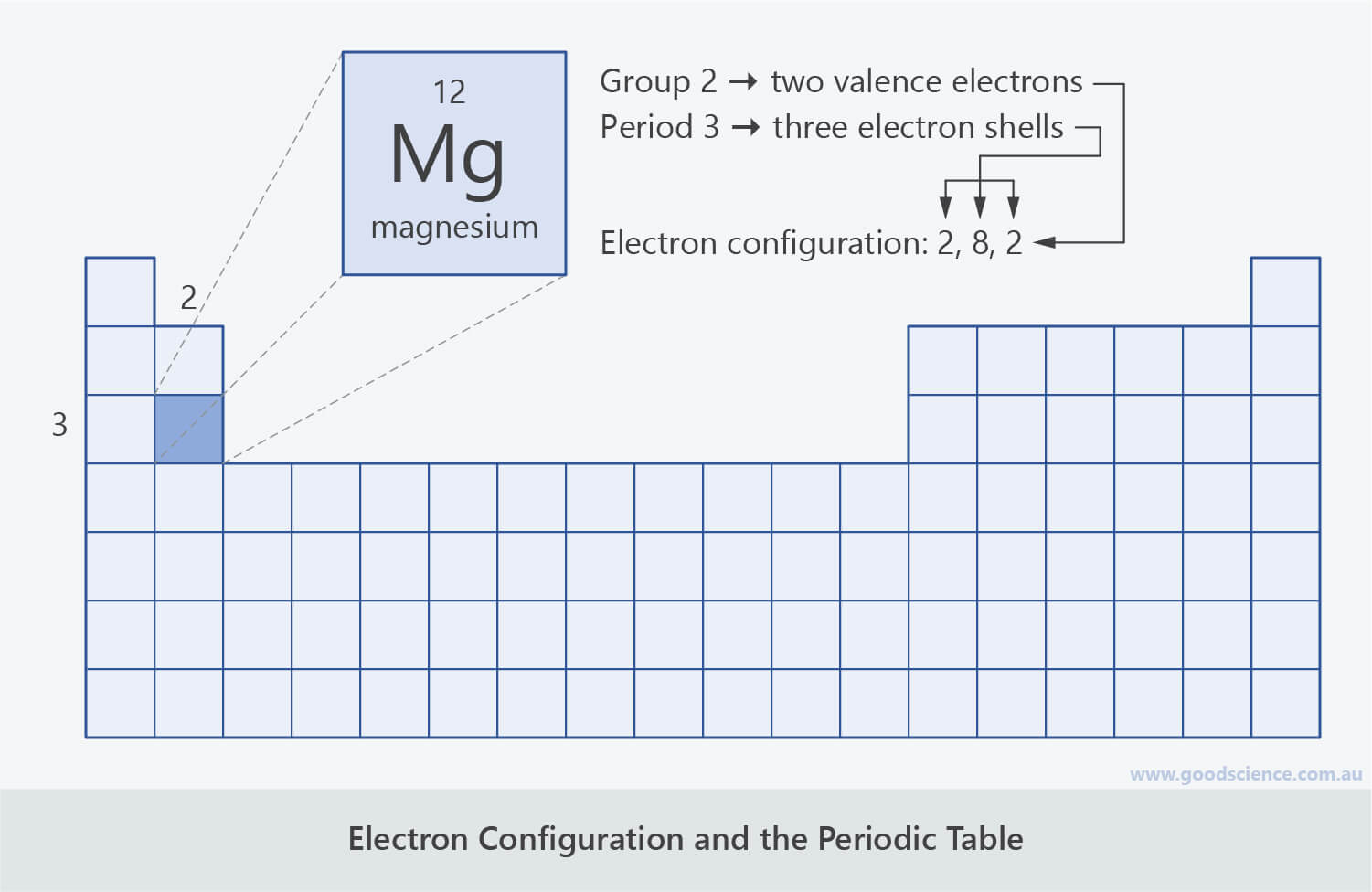
The location of an element on the periodic table is related to its electron configuration.
Formation of Ions
- Since elements in the same main group have the same number of valence electrons, those that form ions will form ions with the same charge.
- Group 1 elements lose one electron to form +1 ions.
- Group 2 elements lose two electrons to form +2 ions.
- Group 3 elements lose three electrons to form +3 ions.
- Group 17 elements gain one electron to form -1 ions.
- Group 16 elements gain two electrons to form -2 ions.
- Group 15 elements gain three electrons to form -3 ions.
- Subsequently, ionic compounds formed from elements in the same two groups will have the same ionic formula.
- For example, all ionic compounds formed from group 2 and group 17 elements have the same general formula AB2.
- The charges of transition metal ions cannot be predicted from the periodic table, but they are always positive.
- They can also have more than one possible charge.
- Charges of transition metal ions can be determined from the chemical formula of compounds, or by looking up a valency table.
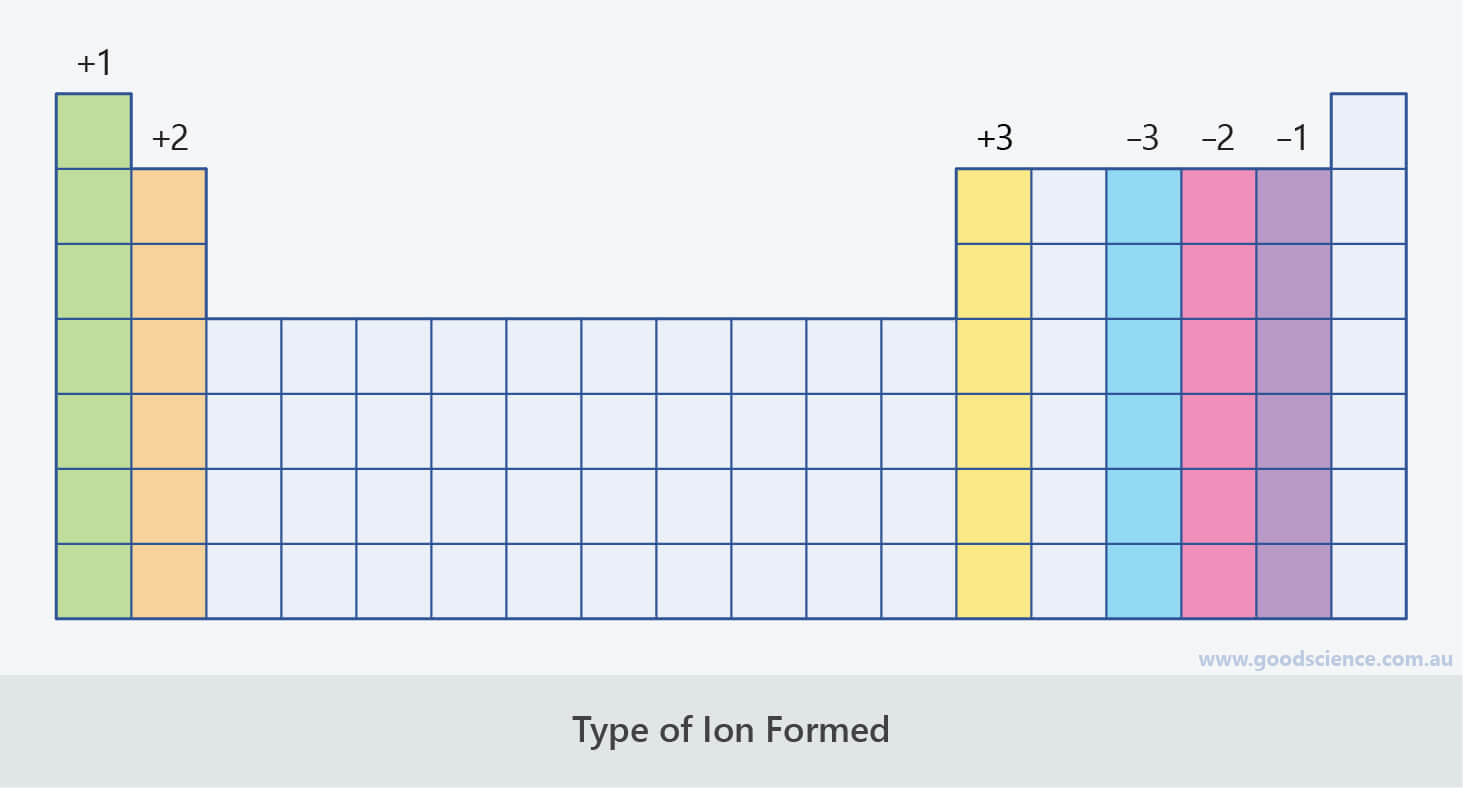
Elements in the same main group form ions with the same charge.

