1 | Overview of the Periodic Table
The Periodic Table of the Elements
- The periodic table of the elements is a way of representing all the known elements.
- Elements are listed in order of increasing atomic number and arranged into groups and periods.
- This layout reflects patterns in the atomic structure of the different elements.
- It subsequently also reflects trends in physical and chemical properties of the elements.
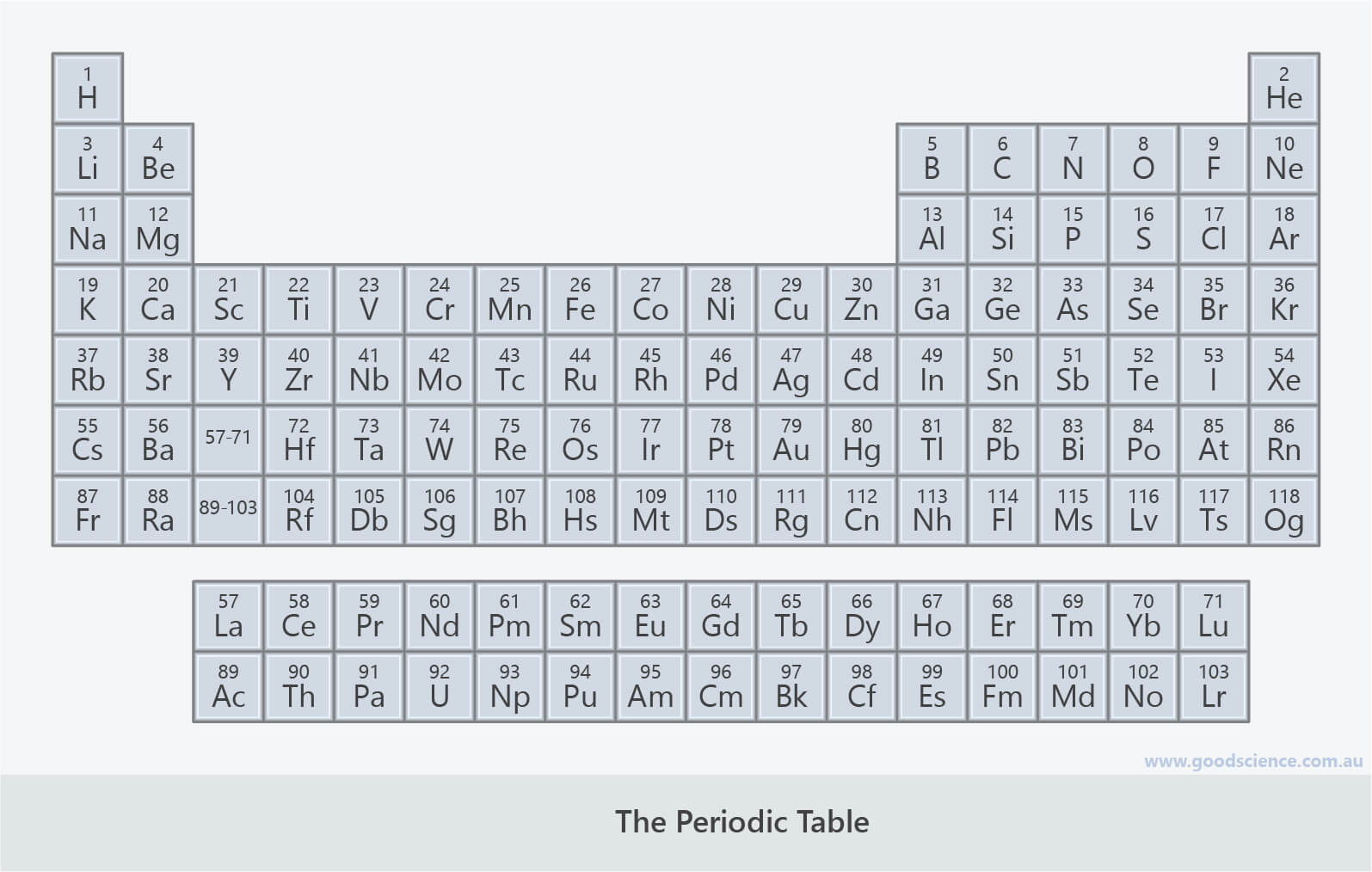
The periodic table contains all the known elements.
Naturally Occurring and Synthetic Elements
- The atomic number of an element is equal to the number of protons in the nuclei of each atom of that element.
- The element with the least number of protons in its atoms is hydrogen – hydrogen atoms contain one proton; therefore hydrogen has an atomic number of 1 and is the first element in the periodic table.
- There are currently 118 known elements, covering atomic numbers 1-118.
- Elements with atomic numbers 1-94 are naturally occurring.
- Elements with atomic numbers greater than 94 are synthetic elements. These elements have only been synthesised artificially, usually in very small quantities.
- All synthetic elements and many of the larger naturally occurring elements are unstable and radioactive.

Elements in the periodic table are arranged in order of increasing atomic number.

