2 | Periods and Groups
Periods
- As elements increase in atomic number, they are arranged into horizontal rows called periods.
- There are seven periods in the periodic table.
- Periods 6 and 7 include two series of elements that are usually listed separately at the bottom of the periodic table.
- These elements are known as the lanthanides and actinides respectively.
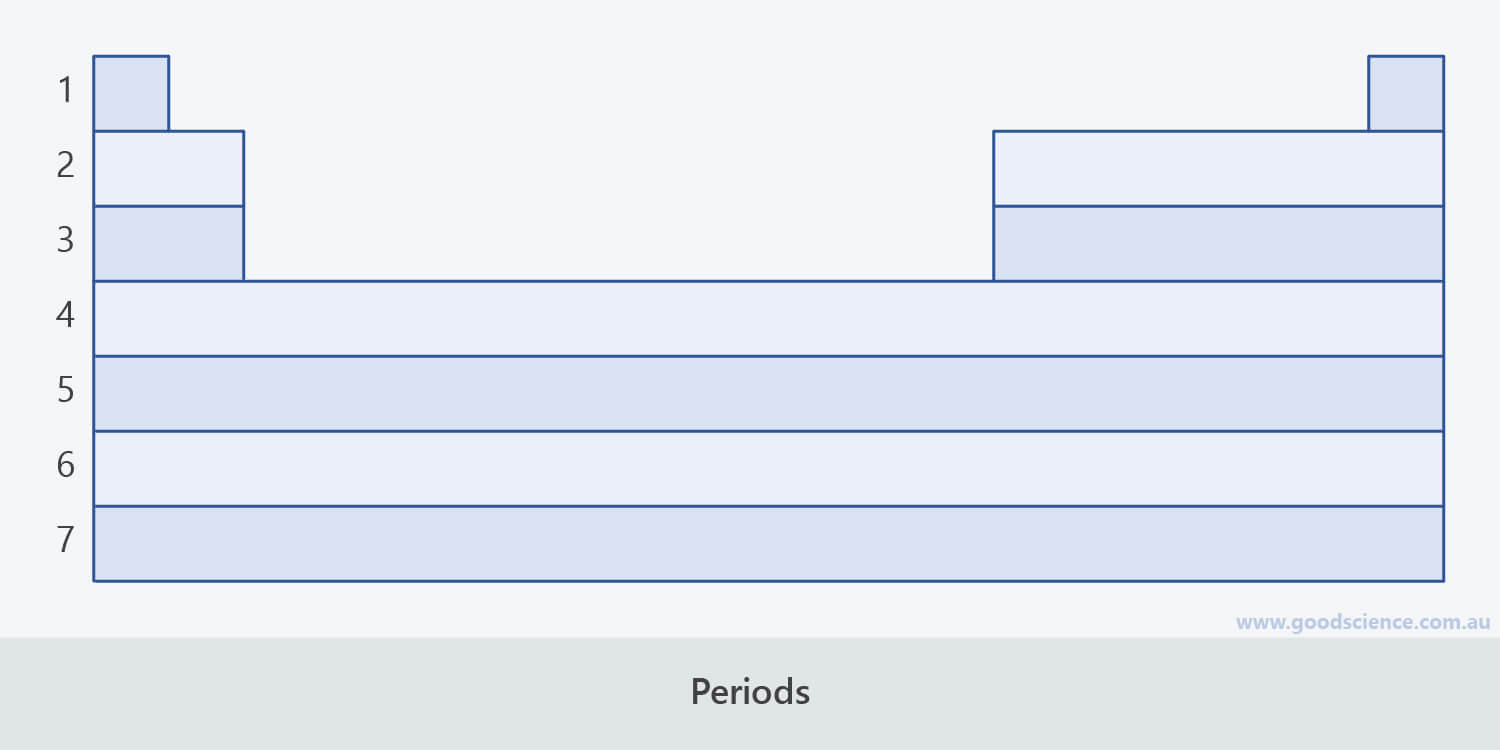
There are seven periods (rows) in the periodic table.
Groups
- The vertical columns of the periodic table are called groups.
- There are 18 groups in the periodic table.
- Groups are numbered 1 to 18.
- Groups 1-2 and 13-18 are sometimes referred to as main group elements.
- Groups 3-12 are known as the transition metals.
- In an older numbering system, Roman numerals were used for groups. The main groups were numbered I-VIII and the transition metal groups had their own numbering system.
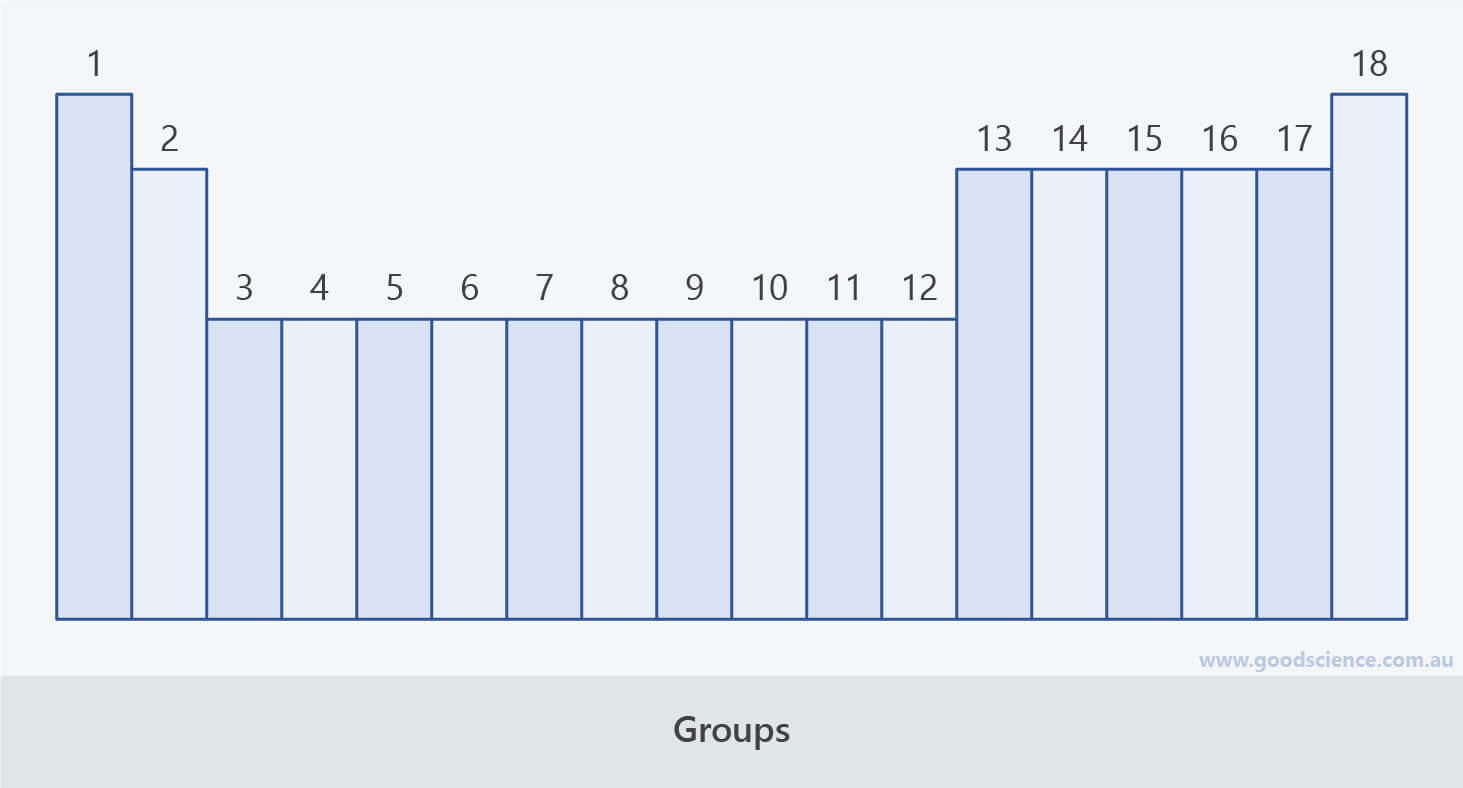
There are 18 groups (columns) in the periodic table.
Arrangement of Groups and Periods
- Groups and periods do not all contain the same numbers of elements.
- This gives the periodic table its unique shape, rather than a simple rectangle.
- The layout of the periodic table arranges groups so that they contain elements with similar physical and chemical properties.
- In fact, as the periodic table was developed, many elements were predicted before they had even been discovered.
- Example 1
- Groups 1 elements (excluding hydrogen) are known as the alkali metals. They are all soft, highly reactive metals that react vigorously with water to form strong alkalis (basic solutions).
- Example 2
- Group 18 elements are known as the noble gases. They are all colourless, odourless gases that are very unreactive.



Lithium, sodium and potassium are alkali metals, which are located in group 1 of the periodic table.
(Images: Dnn87, Wikimedia Commons)
Quizzes

