The Periodic Table and Chemical Bonding
The Periodic Table
10 Topics | 9 Quizzes
Chemical Bonding and Structures
8 Topics | 7 Quizzes
Ionic and Covalent Compounds
8 Topics | 7 Quizzes
Types of Chemical Reactions
Combination, Decomposition and Displacement Reactions
6 Topics | 5 Quizzes
Oxidation Reactions: Combustion and Corrosion
9 Topics | 8 Quizzes
Acid-Base Reactions
10 Topics | 9 Quizzes
Acid-Metal Reactions
5 Topics | 4 Quizzes
Rates of Chemical Reactions
Rate of a Chemical Reaction
4 Topics | 3 Quizzes
Factors that Affect Rate of Reaction
7 Topics | 6 Quizzes
4 | The Alkali Metals

4 | The Alkali Metals
The Alkali Metals
- The metals in group 1 are known as the alkali metals.
- Alkali metals have the following properties:
- They are shiny, silver metals.
- They are soft (can be cut with a knife).
- Although they are solids at room temperature, they have low melting and boiling points (all except lithium have a melting point less than 100°C).
- They have low densities (lithium, sodium and potassium can float on water).
- They are highly reactive (none occur naturally in their elemental form).
- They have one valence electron, which they readily lose to form ions with a charge of +1.
- They react vigorously with water to form strong alkalis (basic solutions) and hydrogen gas.
- They form similar white salts when reacting with group 17 elements.
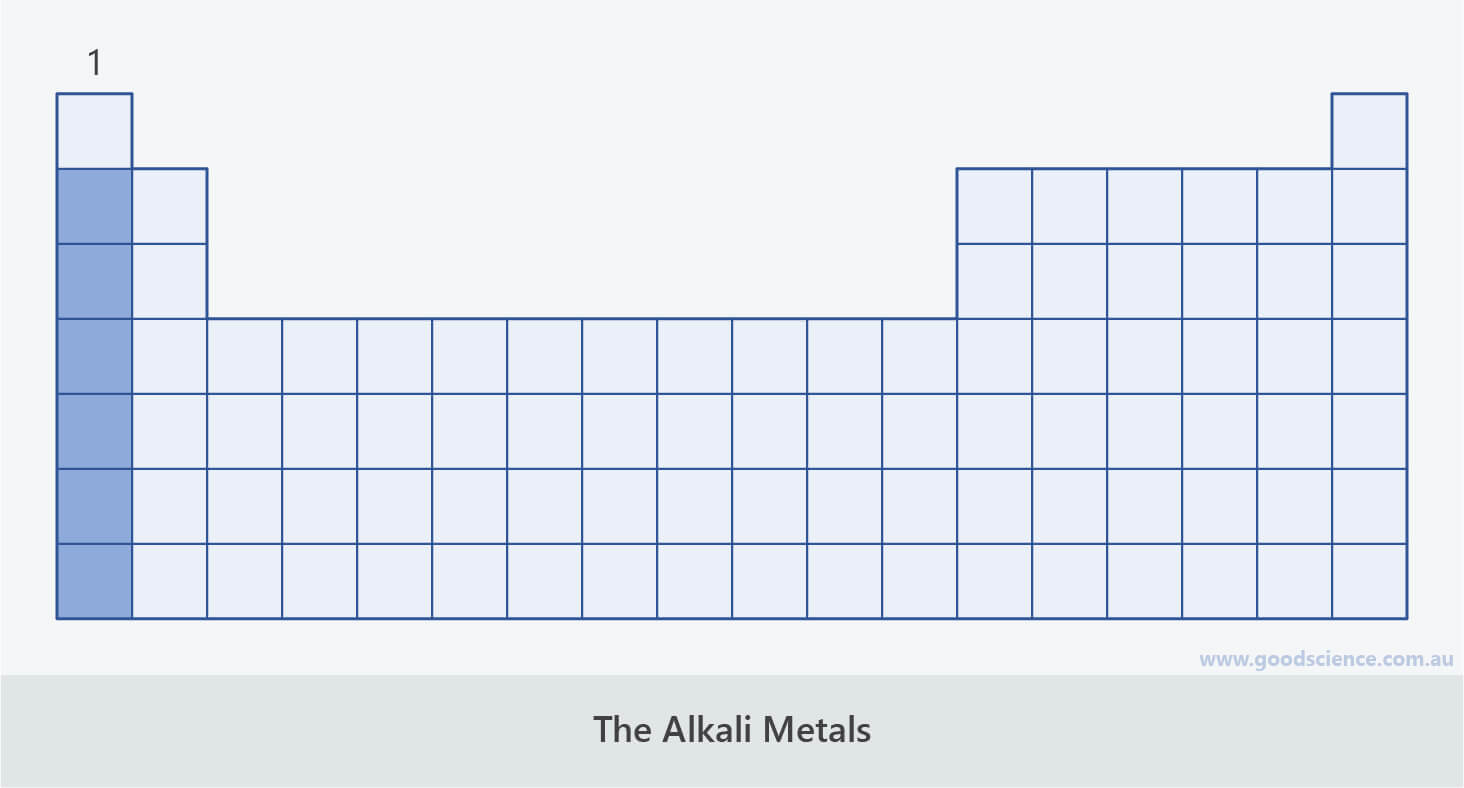
The metals in group 1 are known as the alkali metals.
Quizzes
