The Periodic Table and Chemical Bonding
The Periodic Table
10 Topics | 9 Quizzes
Chemical Bonding and Structures
8 Topics | 7 Quizzes
Ionic and Covalent Compounds
8 Topics | 7 Quizzes
Types of Chemical Reactions
Combination, Decomposition and Displacement Reactions
6 Topics | 5 Quizzes
Oxidation Reactions: Combustion and Corrosion
9 Topics | 8 Quizzes
Acid-Base Reactions
10 Topics | 9 Quizzes
Acid-Metal Reactions
5 Topics | 4 Quizzes
Rates of Chemical Reactions
Rate of a Chemical Reaction
4 Topics | 3 Quizzes
Factors that Affect Rate of Reaction
7 Topics | 6 Quizzes
8 | The Noble Gases

8 | The Noble Gases
The Noble Gases
- The elements in group 18 are known as the noble gases (formerly, the inert gases).
- The noble gases have the following properties:
- They are non-metals.
- They exist as individual atoms.
- They have very low melting and boiling points (all are gases at room temperature).
- They have full valence shells (all have eight valence electrons, except helium, which has two).
- They are chemically very unreactive (only xenon, krypton and argon have been found to form compounds).
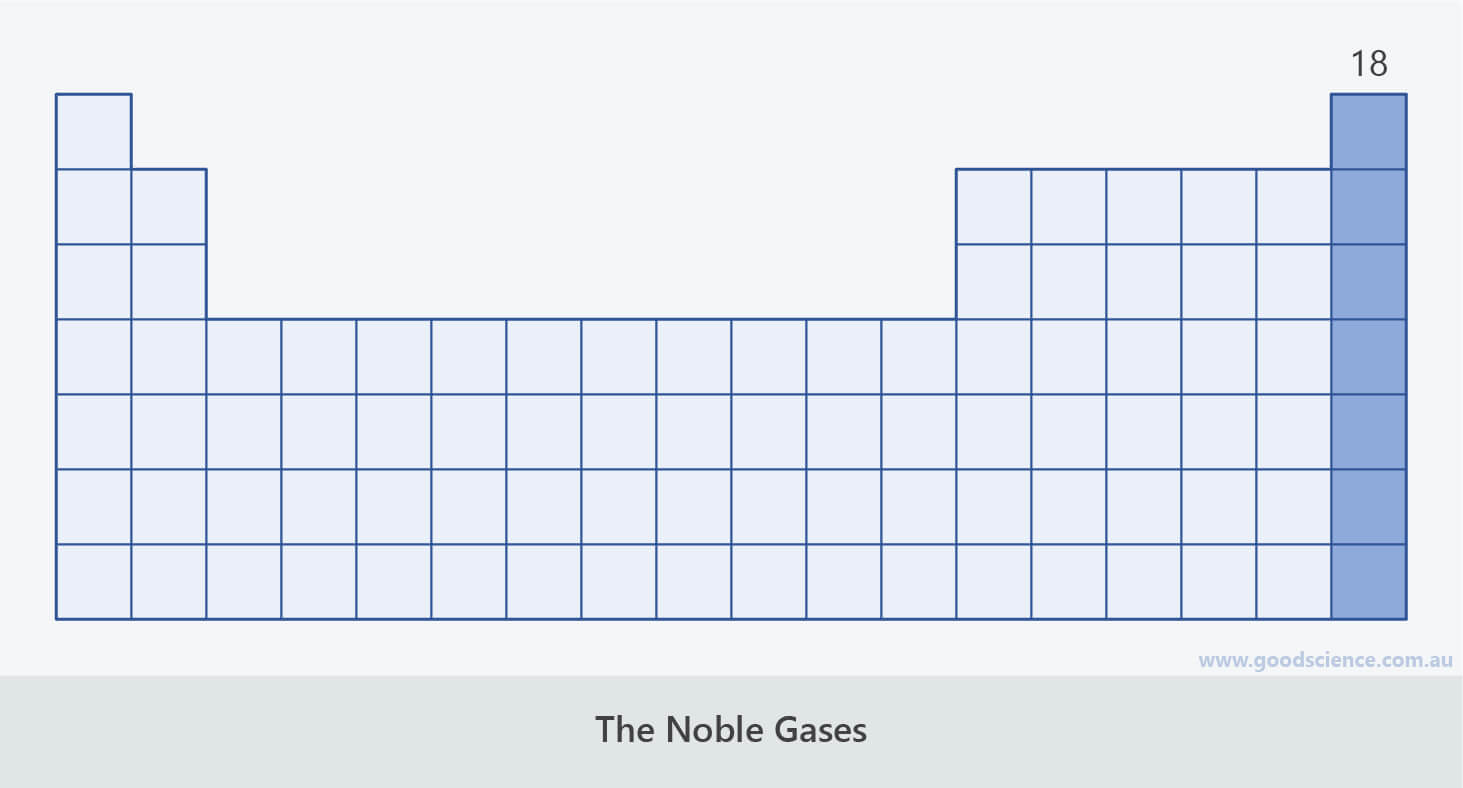
The elements in group 18 are known as the noble gases.
Quizzes
