4 | Density of Solids, Liquids and Gases
Density of Solids, Liquids and Gases
- Density refers to how heavy something is relative to its volume.
- Imagine a block of concrete (a solid), a bucket of water (a liquid) and a balloon (representing the gas inside the balloon), all of which are the same size (volume). The block of concrete and bucket of water would be quite heavy, but the balloon would be very light. We can therefore say that the concrete and water have high density whereas the air has a very low density.
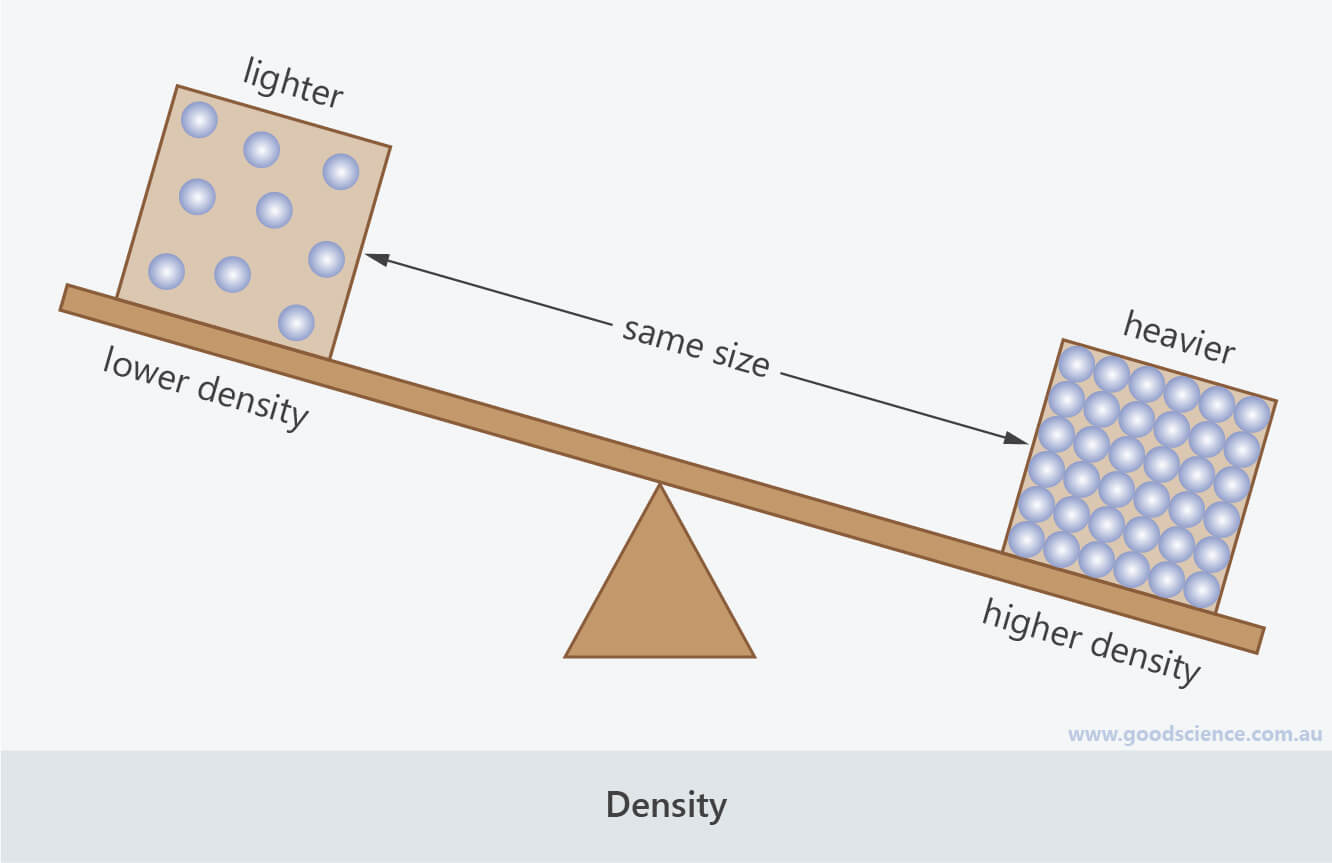
Density refers to how heavy something is, relative to the amount of space it takes up.
Solids
- Solids have a high or very high density (are ‘heavy’ or ‘very heavy’).
- The density of solids can vary.
- For example, most metals are much denser than ice. A block of iron the same size as a block of ice would be almost eight times heavier.
- Some solids, such as styrofoam, may appear very light, but these mostly consist of pockets of air, which is a gas.

Solids have a high density.
(Image: schneich, Pixabay)
Liquids
- Liquids have a high density (are ‘heavy’).
- Similar to solids, the density of liquids can vary, but not as widely.
Liquids have a high density.
(Image: Paul Hamilton, Wikimedia Commons)
Gases
- Gases have a very low density (are ‘very light’).
- Gases can have different densities, but all are very light compared to liquids and solids.

Gases have a very low density.
(Image: stux, Pixabay)

