5 | Electron Configuration
Electron Configuration
- The arrangement of electrons in shells around the nucleus of an atom is referred to as the electron configuration.
- Electron shells fill up from the inner shell first.
- The further away from the nucleus a shell is, the larger it is and the more electrons it can hold.
- The first shell (closest to the nucleus) can hold a maximum of 2 electrons.
- The second shell can hold a maximum of 8 electrons.
- The third shell can hold a maximum of 18 electrons, but once it has 8 electrons, the fourth shell begins to fill.
- For example, even though shell 3 can hold a maximum of 18 electrons, potassium has 8 electrons in the third shell and 1 electron in the fourth shell.
- The maximum number of electrons that can exist in a shell is summarised by the following formula, where n is the shell number (energy level).

Shorthand Electron Configuration
- The electron configuration of an element can be written in an abbreviated form, showing the numbers of electrons in each shell.
- Example
- A silicon atom has 14 electrons. There are two electrons in the first shell, eight electrons in the second shell and four electrons in the third shell. The electron configuration of silicon can therefore can be written as 2,8,4.
![]()
Silicon has the electron configuration 2,8,4.
Valence Electrons
- The outermost electron shell is known as the valence shell.
- It can hold a maximum of 8 electrons, regardless of which shell number it is; the only exception is when the valence shell is the only shell – in this case, the maximum number of electrons is 2.
- Electrons in the valence shell are known as valence electrons.
- In the example above, silicon has four valence electrons.
- The number of valence electrons in an atom largely determines the chemical properties of an element.
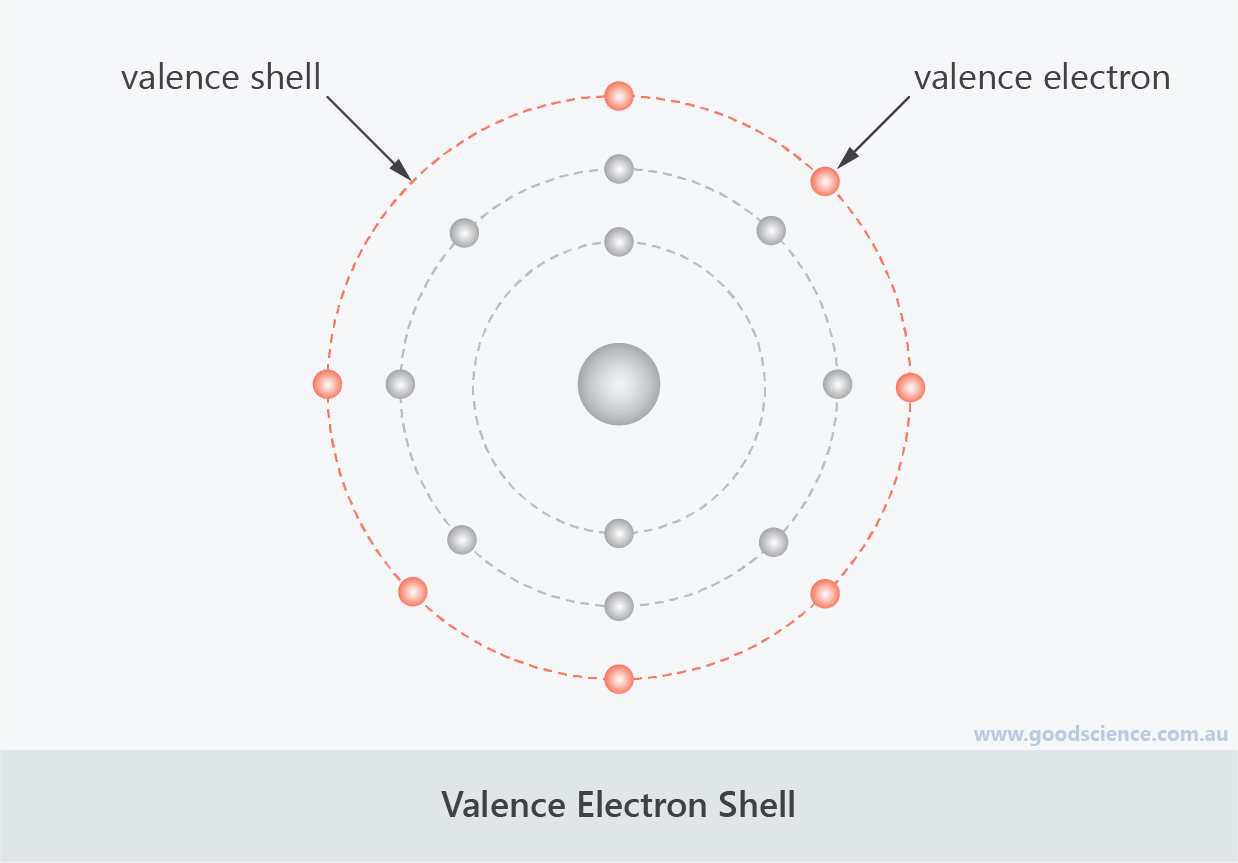
Valence electrons determine an element’s chemical stability.