9 | Trends in the Periodic Table
Trends in the Periodic Table
- Although elements in the same group have similar properties, there are gradual changes in physical and chemical properties going groups.
- There are also gradual changes in physical and chemical properties across periods.
- For example, there are general trends in size of atoms and reactivity of elements.
Trends in Size of Atoms
- As you go down a group, an extra electron shell is added with each period.
- Therefore the size of atoms increases down a group.
- As you go across a period, an extra proton and electron is added with each group.
- These electrons are added to the same shell.
- The extra protons and electrons create greater attraction between nuclei and electrons, pulling the electrons slightly closer to nuclei.
- Therefore the size of atoms decreases across a period.
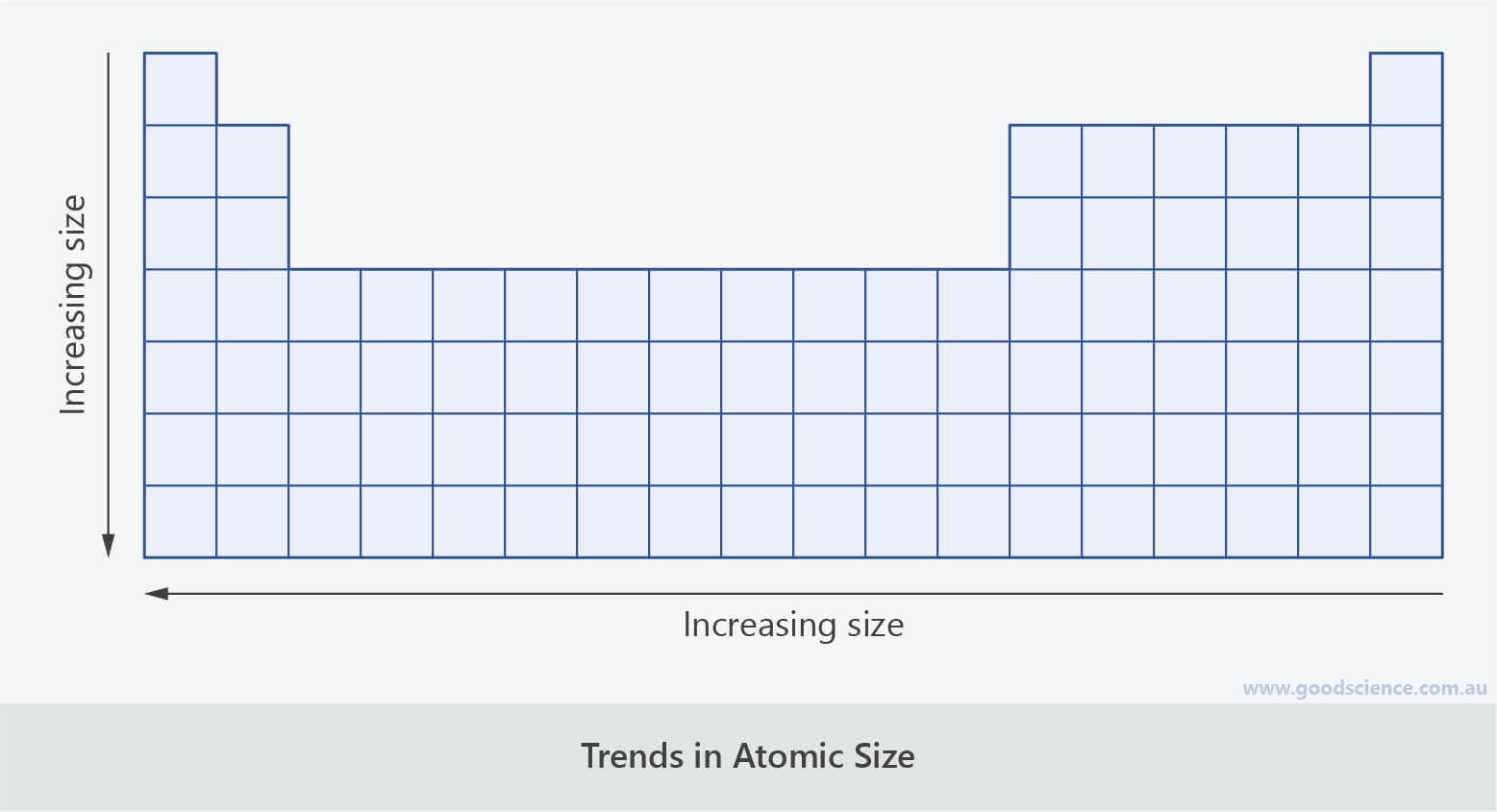
The size of atoms increases down a group and decreases across a period.
Trends in Chemical Reactivity
- Since the size of atoms increases down a group, valence electrons become further away from the nucleus.
- Being further away from the nucleus means the electrons are held less tightly – it’s easier for them to be lost and harder for more to be gained.
- Metals react by losing valence electrons.
- The easier it is to lose them, the more reactive metals are.
- Therefore, since valence electrons are lost more easily down a group, the reactivity of metals increases down a group.
- Non-metals react by gaining electrons.
- The easier it is to gain them, the more reactive non-metals are.
- Therefore, since valence electrons are gained less easily down a group, the reactivity of non-metals decreases down a group.
- Because it is easier to gain or lose one electron than it is to gain or lose two or three electrons, the closer an atom is to having a full valence shell, the more reactive it is.
- Therefore, the reactivity of both metals and non-metals decreases towards the centre of the periodic table (excluding transition metals and noble gases).
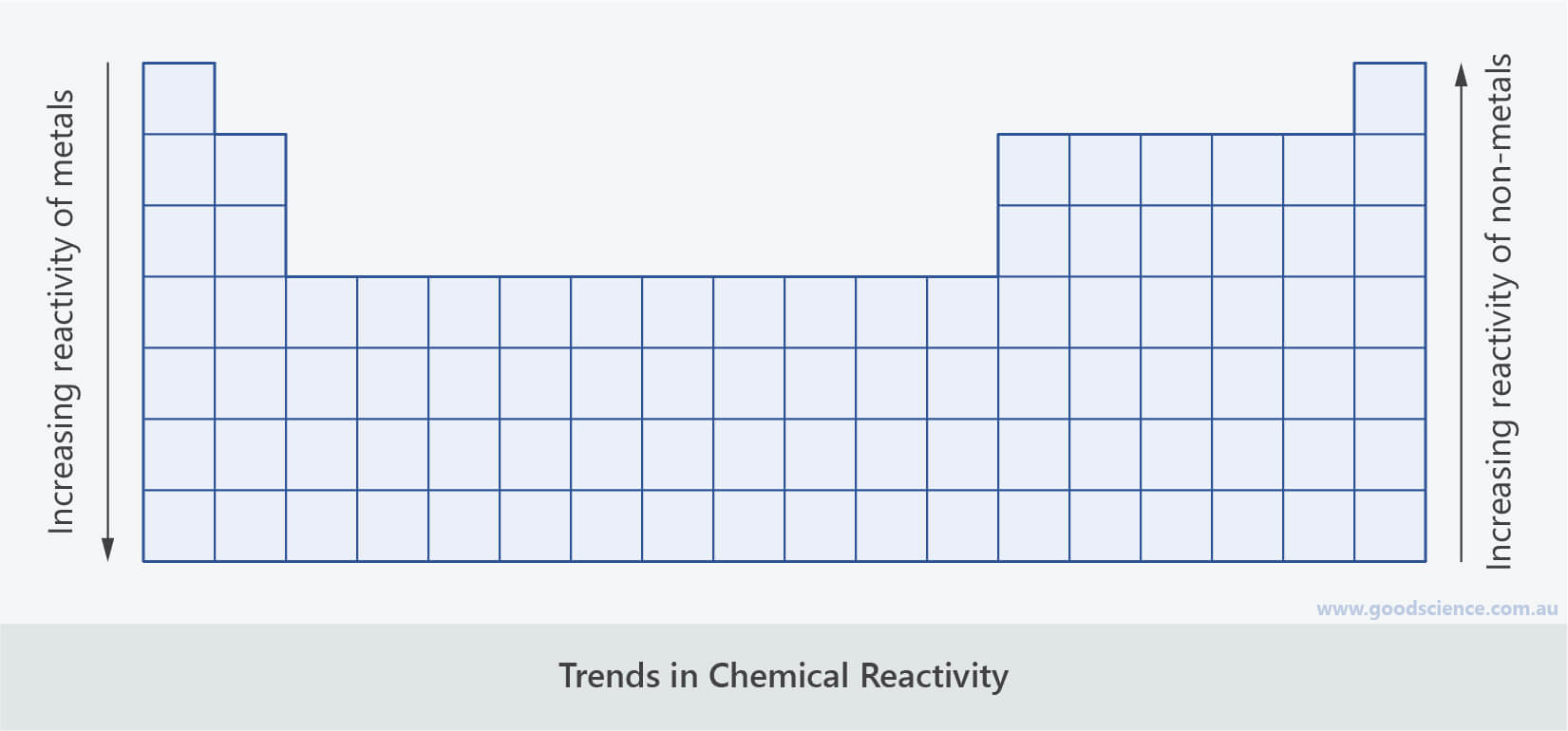
The reactivity of metals increases down a group.
The reactivity of non-metals decreases down a group.

