Properties of Solids, Liquids and Gases
6 Topics | 5 Quizzes
Particles in Solids, Liquids and Gases
8 Topics | 7 Quizzes
Changes in State of Matter
12 Topics | 11 Quizzes
Expansion and Contraction in Solids, Liquids and Gases
5 Topics | 4 Quizzes
Density of Solids, Liquids and Gases
7 Topics | 6 Quizzes
Elements, Compounds and Mixtures
6 Topics | 5 Quizzes
Names and Symbols for Elements and Compounds
4 Topics | 3 Quizzes
Introduction to the Periodic Table
5 Topics | 4 Quizzes
Physical and Chemical Changes
7 Topics | 6 Quizzes
Describing Chemical Reactions Using Equations
4 Topics | 3 Quizzes
Investigating Chemical Reactions
9 Topics | 8 Quizzes
Properties of Solids, Liquids and Gases

Properties of Solids, Liquids and Gases
Learning Objective
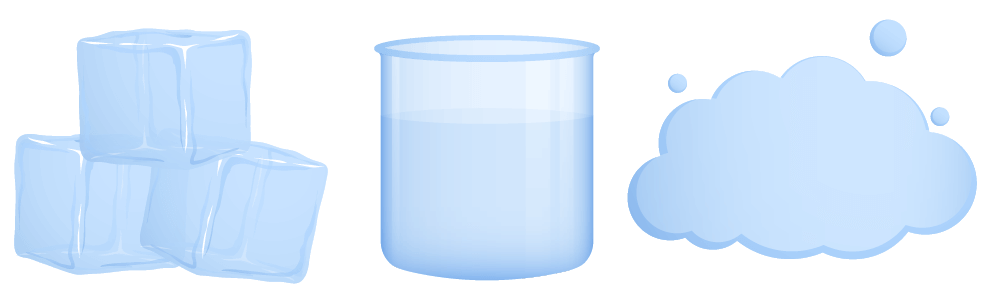
In this lesson we will learn about the three main states of matter and the properties that characterise them.
Learning Outcomes
By the end of this lesson you will be able to:
- Describe properties of solids, liquids and gases.
- Describe the different states of water.
(Image: MicroOne, Adobe Stock)
(Header image: jonathanrostedt, Pixabay)
Lesson Content
0% Complete
0/6 Steps
